“With the CAM Patch, we’ve been able to detect arrhythmias that were totally undetectable by prior Holter or other patch monitors.”
-Samer Ajam, MD
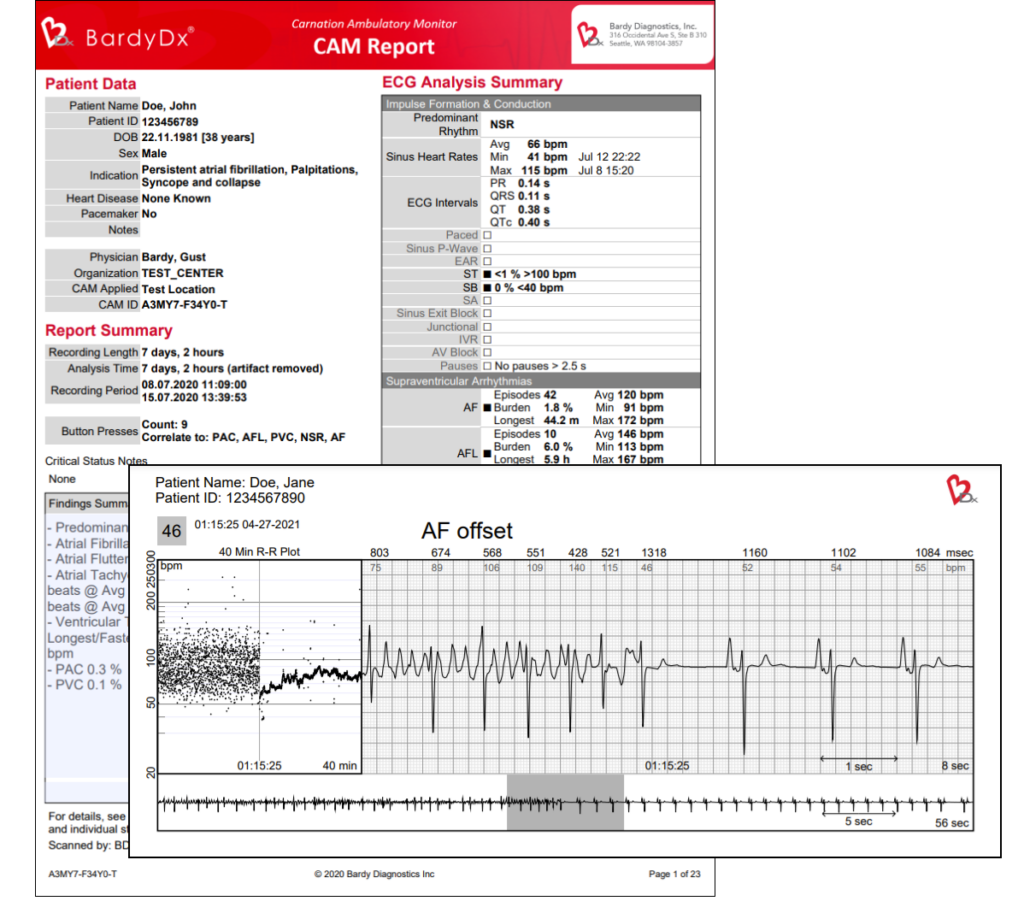
An accurate diagnosis starts with an accurate ECG tracing. The Carnation Ambulatory Monitor (CAM) is a lightweight, extended-wear cardiac patch monitor that delivers clarity,3 convenience, and comfort.1
Developed on the principle that ambulatory cardiac monitoring should provide an excellent quality atrial signal, the CAM Patch is engineered to optimize P-wave signal capture.1
What Healthcare Professionals are Saying
“The CAM has been an incredible step forward in rhythm monitoring for my patients”
A patient management portal that adapts to meet the unique needs of your practice or institution. Upload patient data to the cloud and our expert ECG technicians will analyze the data and provide a full report.
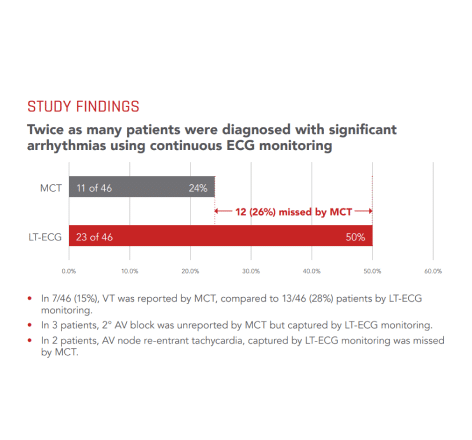
Continuous ECG monitoring versus mobile telemetry: A comparison of arrhythmia diagnostics in human- versus algorithmic-dependent systems.
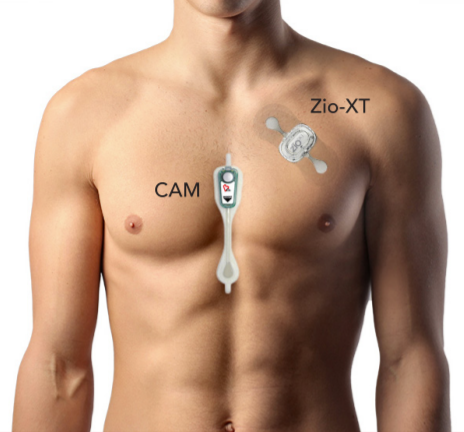
Comparison of two ambulatory patch ECG monitors: The benefit of the P-wave and signal clarity.
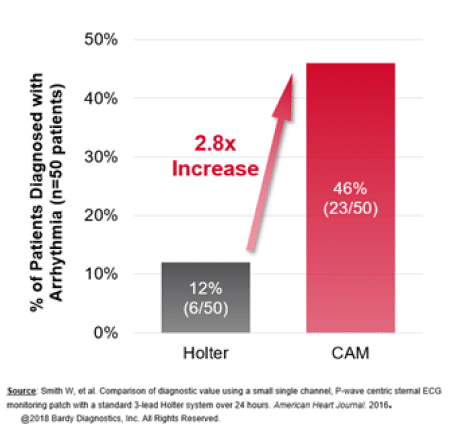
Comparison of diagnostic value using a small, single channel, P-wave centric sternal ECG monitoring patch with a standard 3-lead Holter system over 24 hours.
American Heart Journal 2017
Click here to read more
US-FLC199-220053 (v7.0) 01/2026
Baxter, BardyDx, Bardy Diagnostics, BDx Design and CAM are trademarks of Baxter International Inc. or its subsidiaries.
Any other trademarks, product names or brand images appearing herein are the property of their respective owners.
© 2025 Bardy Diagnostics, Inc. All rights reserved.
Privacy | US Privacy Policy |Terms & Conditions | Intellectual Property | Cookies