BELLEVUE, WA — July 29, 2021– A recent clinical study from Overlake Medical Center utilizing the Bardy Diagnostics Carnation Ambulatory Monitor (CAM) patch was presented at the 2021 Heart Rhythm Society (HRS) Conference. The abstract, “Accelerating Proper Evaluation of Emergency Department Patients for Arrhythmia Concerns with Discharge Use of ECG Patch Monitors,” authored by Marie Yabut, Sarahi Gomes-Duarte, MA., and Eric Shipley, MD, MBA., explores the role of the CAM ECG monitor as a solution for outpatient evaluation after presenting symptoms of cardiac arrhythmias with an inconclusive diagnosis in the emergency department. The decision to either admit or discharge patients for outpatient evaluation is typically arbitrary and there are few alternatives. The abstract concludes that emergency department adoption of ECG monitoring provided an alternative to hospital admission while providing a diagnosis 3 times sooner than average and dramatically reducing return visits to the emergency department.
By utilizing the CAM patch on 412 patients over the 2-year period, the average time of emergency department discharge to diagnosis was 7 days compared to the previous average of 21 days. In addition, emergency department readmissions within 30 days of the initial visit decreased to 7% of study patients compared to readmission of 40% of patients who had the same type of cardiac complaints in the year prior to CAM patch availability.
According to Yabut, using the CAM patch in the emergency department was crucial in providing care for patients with cardiac arrhythmia symptoms occurring outside of normal clinic operating hours. “In the process of this study, we discovered that most patients discharged with the patch occurred outside clinic hours. The availability of the patch in the emergency department benefitted patients who would have otherwise been discharged without a cardiac monitor.” Without application of the CAM patch in the emergency department, patients may experience a substantial waiting period until receiving diagnostic results and proper care.
The study also revealed that using the CAM patch as a point of care solution in the emergency department resulted in new patient referrals to the cardiology/EP department. Between 2018 and 2020, 369 patients were discharged with the CAM patch from the emergency department, and 57 of those patients were then established as cardiac patients. Additionally, 209 patients in the study had no prior cardiology care and were new referrals to Overlake Medical Center’s cardiology/EP department. Due to the diagnostic capabilities of the CAM patch, 178 patients either received further diagnostic testing or a cardiac procedure.
Bardy Diagnostics will be showcasing their CAM patch technology at the 2021 Heart Rhythm Society (HRS) conference in Boston and participating in the conference both virtually and in-person with an exhibitor booth offering visitors an opportunity to explore clinical case studies, experience a new interactive arrhythmia challenge, view a product demo, and discuss clinical issues and trends with the founder of Bardy Diagnostics, Gust H. Bardy, MD. Visit booth #760 at HRS or go to www.bardydx.com/hrs2021.
Source:
Yabut, M., Gomez-Duarte, S. & Shipley, E. (2021, February 11). Accelerating Proper Evaluation Of Emergency Department Patients For Arrhythmia Concerns With Discharge Use Of ECG Patch Monitors. Overlake Medical Center, Bellevue, WA; cOASIS, The Online Abstract Submission and Invitation System.
About Bardy Diagnostics
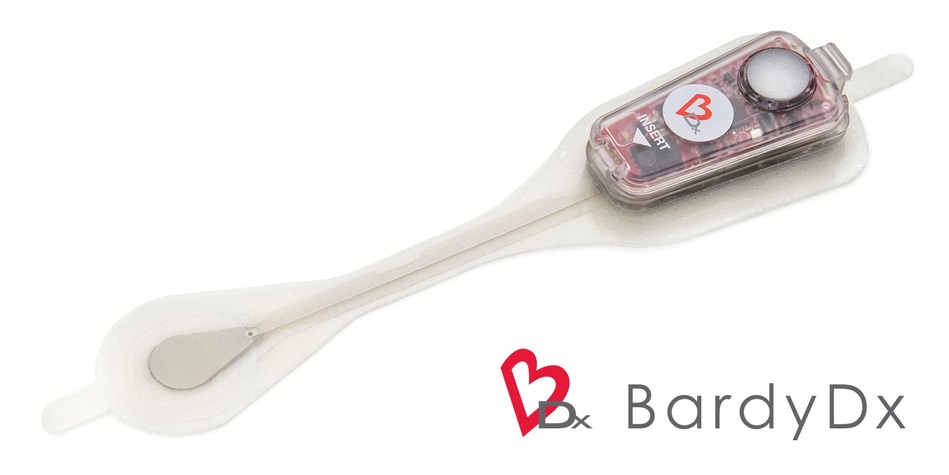
Bardy Diagnostics, Inc. is an innovator in digital health and remote patient monitoring, with a focus on providing the most diagnostically accurate and patient-friendly cardiac patch monitors to the industry. The company’s CAM patch is a non-invasive, P-wave centric™ ambulatory cardiac monitor and arrhythmia detection device that is designed to improve patient compliance for adults and children through its lifestyle-enabling form factor. Designed to be worn comfortably and discreetly for up to 14 days, the female-friendly, hourglass-shaped CAM patch is placed on the center of the chest, directly over the heart for optimum ECG signal collection. The proprietary technology of the CAM patch provides optimal detection and clear recording of the often difficult-to-detect P-wave, the signal of the ECG waveform that is essential for accurate arrhythmia diagnosis. For more information, please visit www.bardydx.com.
About Overlake Medical Center & Clinics
Overlake Medical Center & Clinics is a nonprofit regional healthcare system based in Bellevue serving the Eastside community since 1960. The health system includes a 349-bed hospital and a growing network of primary, urgent and specialty care clinics located throughout the region. Overlake is recognized locally and nationally for quality and safety, including recurring Leapfrog A ratings and Healthgrades’ Patient Safety Excellence Award in 2019 and 2020. Overlake offers comprehensive advanced services including a dedicated Cancer Center, Level lll Trauma Center, Childbirth Center and Level lll NICU, cardiac, neurosciences, orthopedic and mental health services. As part of Overlake’s commitment to deliver high-quality care and exceptional patient experience for the Eastside community, Overlake collaborates with EvergreenHealth in cardiac services, neurosciences and quality, and with Seattle Cancer Alliance for cancer services. Overlake employs more than 3,000 and is dedicated to its mission of compassionate care for every life it touches. Overlake provided nearly $48 million in charity care over the last three years and is committed to providing exceptional patient care and services. For more information, visit overlakehospital.org.
About Heart Rhythm 2021
The Heart Rhythm Society’s annual meeting attracts more than 6,000 of the world’s finest clinicians, scientists, researchers, and innovators in the field of cardiac pacing and electrophysiology. Heart Rhythm 2021 attendees were able to determine how to participate – virtually or in-person. More than 600 international experts in the field will serve as faculty for programing that includes Daily Plenary Sessions, Late-Breaking Clinical Trials, Recorded Cases, Debates, Rhythm Theater Presentations and more, while over 100 exhibitors will showcase innovative products and services.
Related Links